Answer: The osmotic pressure of the solution is 7.44 atm
Step-by-step explanation:
To calculate the concentration of solute, we use the equation for osmotic pressure, which is:
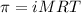
or,
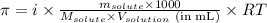
where,
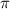 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?
i = Van't hoff factor = 1 (for non-electrolytes)
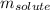 = given mass of glucose = 11.9 g
= given mass of glucose = 11.9 g
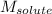 = molar mass of glucose = 180.2 g/mol
= molar mass of glucose = 180.2 g/mol
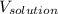 = Volume of solution = 217 mL
= Volume of solution = 217 mL
R = Gas constant =
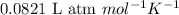
T = temperature of the solution = 298 K
Putting values in above equation, we get:
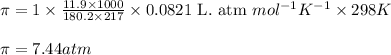
Hence, the osmotic pressure of the solution is 7.44 atm