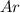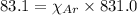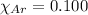Answer: The mole fraction of
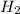 ,
,
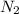 and
and
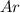 are 0.4950 , 0.4040 and 0.100 respectively.
are 0.4950 , 0.4040 and 0.100 respectively.
Step-by-step explanation:
According to Dalton's law, the total pressure is the sum of individual pressures.
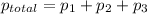
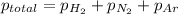
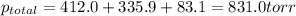
The partial pressure of a gas is given by Raoult's law, which is:
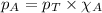 ......(1)
......(1)
where,
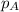 = partial pressure of substance A
= partial pressure of substance A
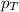 = total pressure
= total pressure
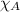 = mole fraction of substance A
= mole fraction of substance A
a) mole fraction of
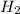
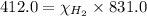
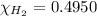
b) mole fraction of
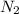
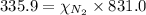
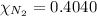
c) mole fraction of