Answer:
The molar entropy of the evaporation of Trichlorofluoromethan is 83.516 J/molK.
Step-by-step explanation:
Entropy :It is defined as amount of energy which is unable to do work or the measurement of randomness or disorderedness in a system.
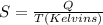
Molar heat of molar vaporization of Trichlorofluoromethane = 24.8 kJ/mol
Temperature at which Trichlorofluoromethan boils , T= 296.95 K
The molar entropy of the evaporation of Trichlorofluoromethan :
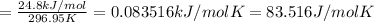
The molar entropy of the evaporation of Trichlorofluoromethan is 83.516 J/molK.