Answer:
The heat needed to boil 1 gallon of water is 81,490.62 Joules.
Step-by-step explanation:
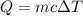
Where:
Q = heat absorbed or heat lost
c = specific heat of substance
m = Mass of the substance
ΔT = change in temperature of the substance
We have :
Volume of water = V = 1 gal = 4546.09 mL
Density of water , d= 1 g/mL
mass of water = m = d × V = 1g/mL × 4546.09 mL = 4546.09 g
Specific heat of water = c = 1 Cal/g°C
ΔT = 100°C - 25°C = 75 °C
9 (boiling pint of water is 100°C)
Heat absorbed by the water to make it boil:
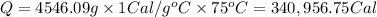
1 calorie = 4.184 J
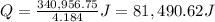
The heat needed to boil 1 gallon of water is 81,490.62 Joules.