One of the fundamental pillars to solve this problem is the use of thermodynamic tables to be able to find the values of the specific volume of saturated liquid and evaporation. We will be guided by the table B.7.1 'Saturated Methane' from which we will obtain the properties of this gas at the given temperature. Later considering the isobaric process we will calculate with that volume the properties in state two. Finally we will calculate the times through the differences of the temperatures and reasons of change of heat.
Table B.7.1: Saturated Methane
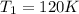
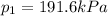
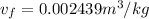
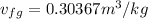
Calculate the specific volume of the methane at state 1
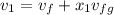
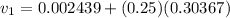
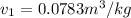
Assume the tank is rigid, specific volume remains constant
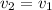
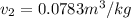
Now from the same table we can obtain the properties,
At
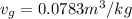
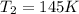
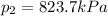
We can calculate the time taken for the methane to become a single phase
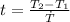
Here
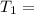 Initial temperature of Methane
Initial temperature of Methane
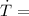 Warming rate
Warming rate
Replacing
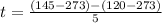
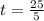
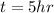
Therefore the time taken for the methane to become a single phase is 5hr