The question is incomplete. the complete question is:
A chemistry student needs 35.0 g of pentane for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of pentane is
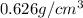 . Calculate the volume of pentane the student should pour out.Round your answer to 3 significant digits.
. Calculate the volume of pentane the student should pour out.Round your answer to 3 significant digits.
Answer: The volume of pentane the student should pour out is 55.9 ml
Step-by-step explanation:
Density is defined as the mass contained per unit volume.
To calculate volume of a substance, we use the equation:
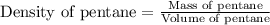
We are given:
Density of pentane =
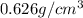
Mass of pentane = 35.0 g
Putting values in above equation, we get:
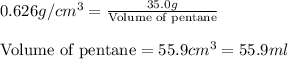
(Conversion factor:
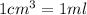 )
)
Hence, the volume of pentane is 55.9 ml