Answer: The percent ionization of HA is 0.13 %
Step-by-step explanation:
We are given:
Molarity of HA solution = 0.10 M
The chemical equation for the ionization of HA follows:
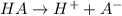
Initial: 0.1
At eqllm: (0.1-x) x x
The expression of
 for above equation follows:
for above equation follows:
![K_a=([H^+][A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/cd33ttbdpkk5h562zi8dnbyn0akiej88j5.png)
We are given:
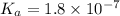
Putting values in above equation, we get:
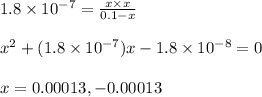
Neglecting the negative value of 'x' because concentration cannot be negative.
To calculate the percent ionization, we use the equation:
![\%\text{ ionization}=([H^+]_(eq))/([HA]_i)* 100](https://img.qammunity.org/2021/formulas/chemistry/college/tco7g7rbsbsaig9pe8fqg8g7zoba418oa6.png)
![[H^+]_(eq)=x=0.00013M](https://img.qammunity.org/2021/formulas/chemistry/college/14vtaz903tqzx2a2ooyle601d530jszxnj.png)
![[HA]_i=0.1M](https://img.qammunity.org/2021/formulas/chemistry/college/wxqh4ikkoc00tssgwmk5zk7yx3q0w0x0um.png)
Putting values in above equation, we get:
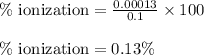
Hence, the percent ionization of HA is 0.13 %