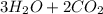Answer:
(a) Two electrochemical half reactions for ethanol
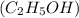 is written below
is written below
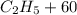 ⇒
⇒
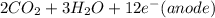
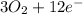 ⇒
⇒
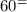
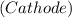
(b) The direct oxidation of the fuel will occur in a solid oxide fuel cell but we have to compete with other sets of chemical electrochemical reaction such as water-gas-shift reaction
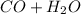 ⇆
⇆
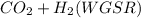
(c) The electrochemical half reaction that could convert ethanol directly into a fuel cell that conducts hydrogen ions is shown below
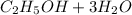 ⇒
⇒
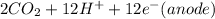
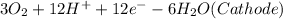
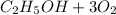 ⇒
⇒
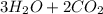
(d) The half reactions that would be required are shown below
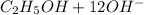 ⇒
⇒
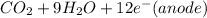
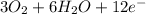 ⇒
⇒
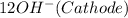
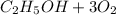 ⇒
⇒