Answer:
The composition of stream on molar basis for glycerol, water and isopropanol is 16.62%, 67.43% and 15.93% respectively.
Step-by-step explanation:
Let suppose the volume is 1
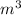 . For three equal components volume of each compound is 0.33
. For three equal components volume of each compound is 0.33
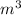 .
.
By using the chemical formulas of the compounds, molecular masses are given as
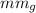 is the molecular mass of glycerol which is 92 g/ mole
is the molecular mass of glycerol which is 92 g/ mole
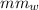 is the molecular mass of water which is 18 g/mole
is the molecular mass of water which is 18 g/mole
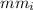 is the molecular mass of isopropanol which is 60 g/mole
is the molecular mass of isopropanol which is 60 g/mole
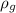 is the density of glycerol which is 1260
is the density of glycerol which is 1260
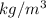
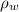 is the density of water which is 1000
is the density of water which is 1000
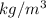
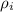 is the density of isopropanol which is 786
is the density of isopropanol which is 786
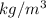
Now using the density and volumes of individual compounds, the masses are given as
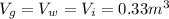
Mass of glycerol is
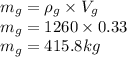
Mass of water is
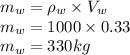
Mass of isopropanol is
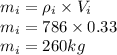
Now number of moles of each compound are given as
Moles of Glycerol are
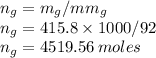
Moles of water are
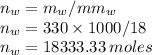
Moles of isopropanol are
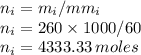
Total number of moles is
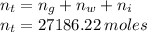
Now composition on molar basis is given as
Molar composition of glycerol is
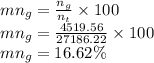
Molar composition of water is
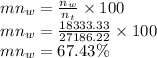
Molar composition of isopropanol is
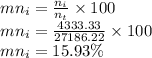
So the composition of stream on molar basis for glycerol, water and isopropanol is 16.62%, 67.43% and 15.93% respectively.