Answer:
The examples of Döbereiner Triad are :
Lithium , Sodium and Potassium
Calcium , Strontium and Barium
Chlorine , bromine and iodine
Sulfur , Selenium and Tellurium
Step-by-step explanation:
Döbereiner Triad : He arranged the element in increasing order of their atomic weight and found that the weight of the middle element is equal to the mean(=average) of the atomic weight of the first and third element .
The example of Döbereiner triad are :
Lithium , Sodium and Potassium
Calcium , Strontium and Barium
Chlorine , bromine and iodine
Sulfur , Selenium and Tellurium
Now, let's know how Döbereiner actually arrived at the result:
1.Lithium , Sodium and Potassium
According to his rule , The mass of Sodium(Middle element) can be calculated :
Mass of Sodium=
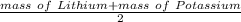
Let's check whether this follow , the Döbereiner triad or not:
mass of Li = 6.94
Mass of Sodium = 23.03
mass of Potassium = 39.10
So according to Döbereiner , the mass of Sodium should be:
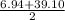
= 22.99
Actual mass = 23 .02
Hence , it is very close to the result. Thus Döbereiner triad is followed by these element.
Similarly for ,
Chlorine , bromine and iodine
mass of Cl = 35.47
mass of iodine = 126.47
So mass of bromine should be ,
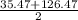
= 70.38
The actual mass of bromine was : 80.47 (almost equal to calculated)
But later it was found that not all elements follow this rule. So this theory was later modified by other scientists.