11. Density of the piece of wood = 3000 g/cm³
12. Density of wooden block = 2 g/cm³. and it will not float in water.
13. Volume of the plastic ball should be 250 mL
14. Mass of the ice cube = 0.162 g
15. Volume of gasoline spilled is equal to 2000 mL
Step-by-step explanation:
11.
Formula:
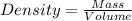
Given:
Mass of wood piece = 7500 g
Volume of wood piece = 2.5 cm³
Therefore,
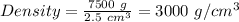
Density of the piece of wood = 3000 g/cm³
12.
Formula:
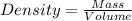
Given:
Dimensions of wooden block = length × width × height = 5 cm × 4 cm × 2 cm
Mass = 80.0 g
Volume of wooden block =
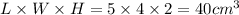
Therefore,
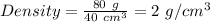
Density of wooden block = 2 g/cm³.
Density of water is equal to 1 g/cm³
2 g/cm³ > 1 g/cm³
Since density of wooden block is greater than the density of water, the piece of wood will not float in water. It will sink.
13.
Formula:
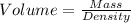
Given:
Mass of the ball = 125 g
Density = 0.5 g/mL
Therefore,
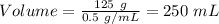
Volume = 250 mL
Volume of the plastic ball should be 250 mL
14.
Formula:
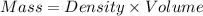
Given:
Dimensions of ice cube = length × width × height = 3 cm × 3 cm × 3 cm
Density = 0.006 g/mL
Volume of ice cube =
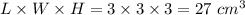
Since,
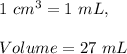
Therefore,
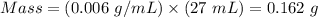
Mass of the ice cube = 0.162 g.
15.
Formula:
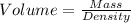
Given:
Mass of gasoline = 1330 g
Density = 0.665 g/mL
Therefore,
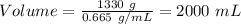
Volume = 2000 mL
Volume of gasoline spilled is equal to 2000 mL