Answer:
36 g
Step-by-step explanation:
Data provided in the question:
A 1 M solution of glucose = 180 g of glucose in a 1 liter solution
i.e Mass of solute = 180 g
Now,
Molarity = [ Number of moles of solute ] ÷ [ Volume Solution in liters ]
also,
Number of moles of solute =
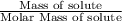
Thus,
1 M =
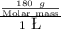
or
Molar mass = 180 g
Therefore,
For molarity = 0.2 M
we have for per liter of solution
0.2 M =
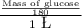
or
Molar glucose = 0.2 × 180
= 36 g