Answer:
a) V1 = 5 ft^3
V2 = 2 ft^3
b) n = 1.378
Step-by-step explanation:
Given data:
mass of gas = 4 lb
starting point
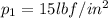
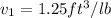
end point
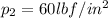
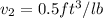
Assume gas to be ideal
a) volume at point 1
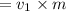
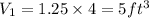
volume at point 2
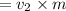

b) from ideal gas equation we have following equation
![(P_1)/(P_2) = [(V_2)/(V_1)]^n](https://img.qammunity.org/2021/formulas/physics/high-school/lcnnjgyzuz5iygbicyha98b7qv855028em.png)
taking log on both side of equation
![ln [(P_1)/(P_2)] = n * ln [(V_2)/(V_1)]](https://img.qammunity.org/2021/formulas/physics/high-school/brvtsoy5xop0lck31pgirr7ndcttu5gj3m.png)
solving for n
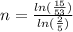
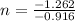
n = 1.378