Answer: y = -x-2
==================================================
Step-by-step explanation:
First we need the slope
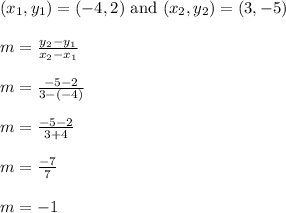
Then we can find the y intercept using the coordinates of (x,y) = (-4,2)
y = mx+b
2 = -1(-4)+b
2 = 4+b
2-4 = b
-2 = b
b = -2
Alternatively, you can use the other point (x,y) = (3,-5) and m = -1.
Because m = -1 and b = -2, we go from y = mx+b to y = -1x+(-2) which simplifies to y = -x-2