Answer:
time required is 193000 s
Step-by-step explanation:
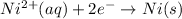
From the above it is clear that for plating out 1 atom of Ni, 2 electrons are needed.
Therefore, for plating out 1 mol of nickel metal, 2 mols of electrons are needed.
Charge of 1 mol of electron = 96500 C = 1 Faraday
Therefore, charge of 2 moles of electron = 2 × 96500
= 193,000 C
Relation between current, charge and time is as follows:
Q = I × t
193000 = 1.00 × t
t = 193000 s