Answer: c) No, using 175 g of sodium chloride in 5 L of solution will give a 3.5% solution.
Step-by-step explanation:
Percent of solution = 35 %
Mass of solute = 175 g
Volume of solution = 5L = 5000 ml (1L=1000ml)
Mass by volume percent (m/v)% : It is defined as the mass of solute present in 100 ml of solution.
Formula used :
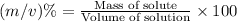
Now put all the given values in this formula:
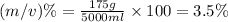
Thus the mass by volume percent is 3.5 % and not 35%.