Answer:
The length of an edge of each small cube is 3.43 nm.
Step-by-step explanation:
Given that,
Temperature of ideal gas =27.0°C
Pressure = 1.00 atm
We need to calculate the length of an edge of each small cube
Using gas equation

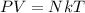
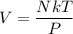
For, N = 1
Where,
N = number of molecule
k = Boltzmann constant
T = temperature
P= pressure
Put the value into the formula
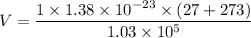
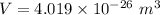
Now, for the cube
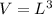
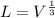
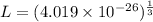
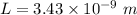
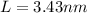
Hence, The length of an edge of each small cube is 3.43 nm.