Answer:
 photons are emitted per second in a
photons are emitted per second in a
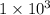 Watt microwave oven.
Watt microwave oven.
Step-by-step explanation:
The expression for the power is:-
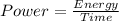
Also,
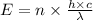
Where,
n = the number of photons
h = Plank's constant having value
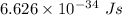
c = the speed of light having value
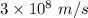
 = the wavelength of the light
= the wavelength of the light
So,
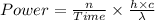
Thus, the expression for photons per second is:-
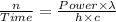
Given that:-
Power = 10³ Watt
( 1 cm = 0.01 m)
[/tex]
So,
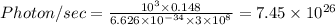
 photons are emitted per second in a
photons are emitted per second in a
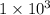 Watt microwave oven.
Watt microwave oven.