Answer:
4883 kPa
Step-by-step explanation:
Standard Otto Cycle pics are attached
Combustion process:
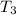 = 2050 K
= 2050 K
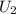 =
=
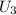 -
-
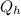
 =
=
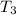 -
-
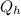 /
/
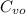
= 2050 - 1000 / 0.717
= 655.3 K
Compression process:
 =
=
 (
(
 /
/
 )^k/(k-1)
)^k/(k-1)
= 90(655.3/290) ^3.5
= 1561 kPa
CR = v1 / v2 = (
 /
/
 ))^1/(k-1)
))^1/(k-1)
= (655.3 / 290) ^2.5
= 7.67
w2 = u2 - u1 =
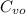 (
(
 -
-
 )
)
= 0.717(655.3 - 290)
= 262 kJ / kg
Highest pressure is after the combustion
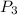 =
=

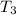 /
/

= 1561 × 2050 / 655.3
= 4883 kPa