Answer:
The answer is C. The partial pressure of hydrogen will be unchanged.
Step-by-step explanation:
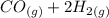 ⇒
⇒
Argon with electronic configuration
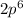
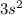
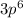 (that is atomic number 18) is an inert gas making it unreactive and it's addition to the reaction has no effect on the partial pressure of either the reactant or production or the state of the system.
(that is atomic number 18) is an inert gas making it unreactive and it's addition to the reaction has no effect on the partial pressure of either the reactant or production or the state of the system.
The partial pressure of hydrogen will remain unchanged on the addition of Argon.