This is an incomplete question, here is a complete question.
Hydrogen and iodine react to form hydrogen iodide, like this:
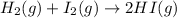
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, iodine, and hydrogen iodide has the following composition:
Compound Pressure at equilibrium
 61.8 atm
61.8 atm
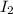 46.5 atm
46.5 atm
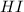 52.3 atm
52.3 atm
Calculate the value of the equilibrium constant
 for this reaction. Round your answer to 2 significant digits.
for this reaction. Round your answer to 2 significant digits.
Answer : The value of equilibrium constant
 for this reaction is, 0.952
for this reaction is, 0.952
Explanation :
The given chemical reaction :
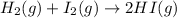
The expression of
 for above reaction follows:
for above reaction follows:
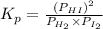
We are given:
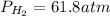
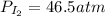
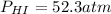
Putting values in above equation, we get:
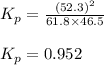
Therefore, the value of equilibrium constant
 for this reaction is, 0.952
for this reaction is, 0.952