Answer:
If it was 1:3, the unknown will be 7.5ml and if it was 1:4, the unknown will be 8ml. The explanation is shown below:
Step-by-step explanation:
Stoichiometric ratio of hypochlorite and unknown
Ratio 1:1 in 10ml
Hypochlorite =
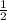 mole fraction = 0.5
mole fraction = 0.5
= 0.5ml x 10ml = 5ml
Unknown = 5ml (i.e. 10ml [total volume given] - the answer of the hypochlorite)
Ratio 1:3
Hypochorite =
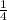 mole fraction x 10ml
mole fraction x 10ml
= 0.25 x 10ml = 2.5ml
Unknown = 10ml - 2.5ml = 7.5ml
Ratio 1:4
Hypochlorite =
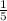 mole fraction x 10ml
mole fraction x 10ml
= 0.2 x 10ml = 2ml
Unknown = 10ml - 2ml = 8ml.