Answer:
moles of glucose
2.3166 moles of glucose
Step-by-step explanation:
The balance reaction for the formation of glucose is :
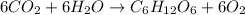
here , CO2 = carbon dioxide
H2O = water
C6H12O6 = glucose
O2 = Oxygen
According to this equation :
6 mole of CO2 = 6 mole of H2O = 1 mole of C6H12O6 = 6 mole of O2
We are asked to calculate the mole of Glucose from carbon dioxide.
So,
6 mole of CO2 produce = 1 mole of C6H12O6
1 mole of CO2 will produce =
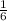 moles of glucose
moles of glucose
13.9 moles of CO2 will produce :

=2.3166 moles of glucose
Note : first , Always calculate for one mole (By dividing)
. After this , multiply the answer with the moles given.
Always write the substance whose amount is asked(glucose) to the right hand side