The question is incomplete, here is the complete question:
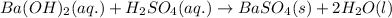
Xianming runs a titration and collects, dries, and weighs the
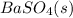 produced in the experiment. He reports a mass of 0.2989 g go
produced in the experiment. He reports a mass of 0.2989 g go
 Based on this, calculate the concentration of
Based on this, calculate the concentration of
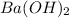 solution.
solution.
Answer: The concentration of barium hydroxide solution is 0.0013 moles.
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of barium sulfate = 0.2989 g
Molar mass of barium sulfate = 47.87 g/mol
Putting values in above equation, we get:
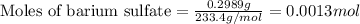
The given chemical reaction follows:
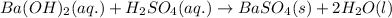
By Stoichiometry of the reaction:
1 mole of barium sulfate is produced from 1 mole of barium hydroxide
So, 0.0013 moles of barium sulfate will be produced from =
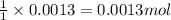 of barium hydroxide
of barium hydroxide
As, no volume of the container is given. So, the concentration will be calculated in moles only.
Hence, the concentration of barium hydroxide solution is 0.0013 moles.