Answer:
1. What is the mass of water displaced by the lead weight?
m = 17.5g
2. What is the volume of water displaced by the lead weight?
V = 17.5mL
3. What is the volume of the lead weight?
V = 17.5mL
4. What is the mass of the lead weight?
m = 199.0g
5. What is the density of the lead weight?
d = 11.30g/mL
Step-by-step explanation:
1. What is the mass of water displaced by the lead weight?
In order to know the mass displaced by the lead weight, the initial grams must be subtracted, in this case 0.0g minus the grams of the suspended lead.
0.0g - 17.5g = 17.5g
2. What is the volume of water displaced by the lead weight?
To know the volume of water displaced, the value of water density must be known, which is 0.9982g / mL. Then the density equation is used, which is:
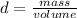
Clearing the volume:
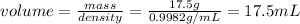
3. What is the volume of the lead weight?
The volume of lead is going to be the same volume of displaced water since, as it says in the initial data, the displaced volume of water is the same volume of submerged lead weight.
4. What is the mass of the lead weight?
The mass of the lead weight will be the mass read on the scale which is 199.0g, because the balance was tared.
5. What is the density of the lead weight?
We use the density equation of question 3, where the mass value is that from question 4 (199.0g) and the volume is found in question 3 (17.5g / mL).
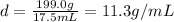
And when we compared with the tabulated value of lead density we see that it perfectly matches.