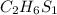Answer:
The empirical formula of the organic compound is =
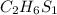
Step-by-step explanation:
At STP, 1 mole of gas occupies 22.4 L of volume.
Moles of
 gas at STP occupying 2.0 L = n
gas at STP occupying 2.0 L = n
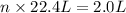
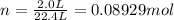
Moles of carbon in 0.08920 mol = 1 × 0.08920 mol = 0.08920 mol
Moles of
 gas at STP occupying 3.0 L = n'
gas at STP occupying 3.0 L = n'
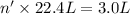
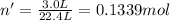
Moles of hydrogen in 0.1339 moles of water vapor = 2 × 0.1339 mol = 0.2678 mol
Moles of
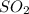 gas at STP occupying 1.0 L = n''
gas at STP occupying 1.0 L = n''
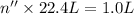

Moles of sulfur in 0.04464 mol = 1 × 0.04464 mol = 0.04464 mol
Moles of carbon , hydrogen and sulfur constituent of that organic compound .
Moles of carbon in 0.08920 mol = 1 × 0.08920 mol = 0.08920 mol
Moles of hydrogen in 0.1339 moles of water vapor = 2 × 0.1339 mol = 0.2678 mol
Moles of sulfur in 0.04464 mol = 1 × 0.04464 mol = 0.04464 mol
For empirical; formula divide the least number of moles from all the moles of elements.
carbon =
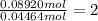
Hydrogen =

Sulfur =

The empirical formula of the organic compound is =