Answer:
A.The surface tension of ethylene glycol is less than water.
Water = Surface tension =more
B.The boiling point of ethylene glycol is more than water.
Ethylene Glycol = Boiling point = more
C.The freezing point of ethylene glycol is less than water.
Freezing Point = Ethylene Glycol = less
D.The viscosity of ethylene glycol is more than water.
Viscosity = Ethylene glycol = More
Step-by-step explanation:
The Chemical formula of ethylene glycol and water are as follow:
 = water
= water
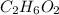 = ethylene glycol
= ethylene glycol
1. Surface Tension : It is defined as the force acting per unit length perpendicular to the line drawn on the surface of the liquid.
It is expressed in the unit of N/m
At 25 degreeC , the surface tension of water is 72 milli N/m
At the same temperature the surface tension of ethylene glycol is 46.3 milliN/m
Hence ethylene glycol surface tension is less than water.The reason for this is the hydrogen bonding.
The H- Bonding is the interaction of more electronegative element (O) with the Hydrogen of the other molecule.
In ethylene glycol the -CH2 CH2 group do not enteract with the O - of the other molecule. Hence the molecule in the bulk can pull (create tension) toward themself. So there is less tension in ethylene Glycol.
But in water more tension is created, since there is no CH2CH2 group in it.
2. Boiling Point : The boiling point of water is : 100 degree
The boiling point of Ethylene Glycol is : 197.3 dergree celcius
Here, in both there are two H- Bonding OH groups present but the molar mass of ethylene glycol is greater than water . This High molar mass increase the boiling point of ethylene glycol.
3.Freezing Point :
The freezing point of ethylene glycol is = -12.9 degree celcius
The frezing point of water is = 0 degree celcius
Hence the freezing point of water is more than ethylene glycol.
4. Viscosity :
The viscosity of water is = 0.00089 Pa s
The viscosity of ethylene glycol = 0.0161 Pa s
Hence Viscosity of Ethylene glycol is more.
It is the resistance to the flow of liquid which arises due to internal friction between molecules.
It is easy to understand viscosity in terms of water and honey.
The flow of honey is slow because it is thick liquid .Hence its viscosity is more.
Similarly , the ethylene glycol is also thick liquid.