Answer:
a. The volume will increase because the decrease in air pressure will have a greater effect than the decrease in temperature.
Step-by-step explanation:
Hello,
In this case, we could predict the resulting volume numerically by using the combined ideal gas relationship as shown below:

Now, the volume as the first state is computed form the ideal gas law:
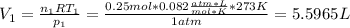
Thus, for the given conditions, we must solve for the volume at the second state:
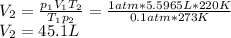
Such volume, clearly is explained by: a. The volume will increase because the decrease in air pressure will have a greater effect than the decrease in temperature.
Best regards.