Answer:
1.32 seconds would take for the concentration of A to decrease from 0.680 M to 0.370 M.
Step-by-step explanation:
Using integrated rate law for first order kinetics as:
![[A_t]=[A_0]e^(-kt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/p2vpxs9cve2w798wzy926mzqzvfv5si8xr.png)
Where,
![[A_t]](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6se0yk0a5jz0ud2m1a9jh5tv0rk9jx59i.png) is the concentration at time t
is the concentration at time t
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) is the initial concentration
is the initial concentration
Given that:
The rate constant, k =
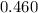 s⁻¹
s⁻¹
Initial concentration
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) = 0.680 M
= 0.680 M
Final concentration
![[A_t]](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6se0yk0a5jz0ud2m1a9jh5tv0rk9jx59i.png) = 0.370 M
= 0.370 M
Time = ?
Applying in the above equation, we get that:-
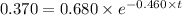
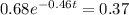
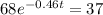
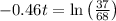
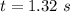
1.32 seconds would take for the concentration of A to decrease from 0.680 M to 0.370 M.