Step-by-step explanation:
More is the intermolecular forces present between the molecules of a substance more will be the boiling point of the substance.
Since, water is able to form hydrogen bonding and it also have dipole-dipole interaction as these are stronger than the forces present in ammonia. Hence, boiling point of water is greater than the boiling point of
 .
.
In
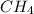 , there are only dispersion forces present in it and these forces are weakest intermolecular forces.
, there are only dispersion forces present in it and these forces are weakest intermolecular forces.
As a result, the enthalpies of vaporization of the following substances increase in the order
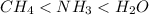 .
.