Answer:
Overall, the formation of sulfuric acid occurs as further:
1.
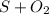 ⇒
⇒
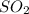 - sulfur containing compound gets oxidized
- sulfur containing compound gets oxidized
2.
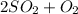 ⇒
⇒
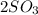 - sulfur dioxide oxidizing to sulfur trioxide
- sulfur dioxide oxidizing to sulfur trioxide
3.
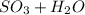 ⇒
⇒
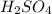 - sulfur trioxide further reacts with water
- sulfur trioxide further reacts with water
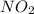 enters the cycle after the first step as a gas. It oxidises the sulfur dioxide, resulting in sulfur trioxide, and itself becomes nitrogen monoxide. However, due to the oxygen in the air. it gets oxidised again and becomes readily available to oxidise another molecule of sulfur dioxide. This process repeats and repeats until all are converted.
enters the cycle after the first step as a gas. It oxidises the sulfur dioxide, resulting in sulfur trioxide, and itself becomes nitrogen monoxide. However, due to the oxygen in the air. it gets oxidised again and becomes readily available to oxidise another molecule of sulfur dioxide. This process repeats and repeats until all are converted.
All reagents in equations 1 and 2 are in gaseous state!
In the 3rd equation, the water is liquid and sulfuric acid itself is aqueous.