Complete question:
Natural gas at 70 ⁰F and standard atmospheric pressure of 14.7 psi (abs) is compressed isentropically to a new absolute pressure of 70 psi. Determine the final density and temperature of the gas.
Answer:
The final density of the natural gas is 0.004243 slugs/ft³ and
The final temperature of the natural gas is 306.6 ⁰F
Step-by-step explanation:
For Ideal gas: P = ρRT
R is ideal gas constant = 3.099 x 10³ ft lb / slug⁰R
T₁ is initial temperature = 70 ⁰F = (70+460)⁰R = 530⁰R
P₁ is intial pressure of the gas = 14.7 psi = (14.7 lb/in² X 144 in²/ft²) = 2116.8 lb/ft²
From the ideal gas equation, we calculate initial density of the natural gas:
ρ₁ = P/RT ⇒ 2116.8/(3.099 x 10³ X 530) = 0.001289 slugs/ft³
For isentropic process:
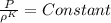
where K is the ratio of specific heats for natural gas; K = 1.31, Therefore
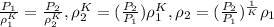
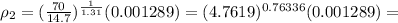 0.004243 slugs/ft³
0.004243 slugs/ft³
Final density; ρ₂ = 4.243 X10⁻³ slugs/ft³
From ideal gas equation; P = ρRT
P₂ = ρ₂RT₂
T₂ = P₂/ρ₂R
P₂ (lb/ft²) = (70 lb/in²)( 144 in²/ft²) = 10080 lb/ft²
T₂ = 10080/(0.004243 X 3099)
T₂ = 766.6⁰R
Final Temperature; T₂ = (766.6-460)⁰F = 306.6⁰F