Answer: Metal A is 3 times faster than Metal B.
Explanation:
Since we have given that
Time taken to oxidised metal A =
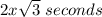
Time taken to oxidised metal B =
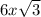 seconds
seconds
So, ratio of metal A to metal B would be
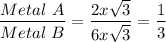
So, Ratio of Metal A to Metal B is 1 : 3.
Hence, Metal A is 3 times faster than Metal B.