Answer: Normal boiling point of the substance is 248 K
Step-by-step explanation:
The vapor pressure is determined by Clausius Clapeyron equation:
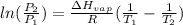
where,
 = initial pressure at normal boiling point= 1 atm (standard atmospheric pressure
= initial pressure at normal boiling point= 1 atm (standard atmospheric pressure
 = final pressure at 371 K= 0.138 atm
= final pressure at 371 K= 0.138 atm
= enthalpy of vaporisation = 12.3 kJ/mol = 12300 J/mol
R = gas constant = 8.314 J/mole.K
 = normal boiling point = ?
= normal boiling point = ?
 = boiling point at pressure of 0.138 atm = 371 K
= boiling point at pressure of 0.138 atm = 371 K
Now put all the given values in this formula, we get
![\log ((1atm)/(0.138atm))=(12300)/(2.303* 8.314J/mole.K)[(1)/(T_1)-(1)/(371)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/h9nkc7i68ann1nbstdug4qbvj8udloyat2.png)
![0.860=(12300)/(2.303* 8.314J/mole.K)[(1)/(T_1K)-(1)/(371K)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/jm7r68m8iccqfr4x1svh0x8fm733b1dbae.png)
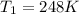
Thus the normal boiling point of the substance in kelvin is 248