Answer:
pH of the solution is 10.37
Step-by-step explanation:
![pOH=pkb+log([salt])/([base])](https://img.qammunity.org/2021/formulas/chemistry/college/kawqztqgxvk6rzxvckgii59os2jiqutff2.png)
kb =
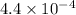
pkb = -log kb
=
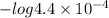
= 3.35
salt is methylammonium bromide and methylamine is base
Substitute the values in the above expression as follows:
![pOH=pkb+log([salt])/([base]) \\=3.35+log(0.35)/(0.18) \\=3.35+0.28\\=3.63](https://img.qammunity.org/2021/formulas/chemistry/college/s98k7xy09pd7gy9vj9zcifvs5bfr333oab.png)
pH = 14 - pOH
= 14 - 3.63
= 10.37
pH of the solution is 10.37