Answer:
It will take 1.59 seconds of time to the concentration of
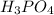 to decrease to 20.0% of its initial value.
to decrease to 20.0% of its initial value.
Step-by-step explanation:
Integrated rate law for second order kinetics is given by:
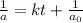
Half life for second order kinetics is given by:
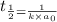
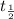 = half life
= half life
k = rate constant
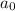 = initial concentration =
= initial concentration =
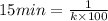
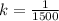
a= concentration left after time t
We have :
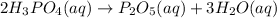
Rate of the reaction, :
![R = (46.6 M^(-1)s^(-1))* [H_3PO_4]^2](https://img.qammunity.org/2021/formulas/chemistry/college/5so4wrxs91fyljevagavmfp6417nykw8it.png)
Order of the reaction = 2
k =
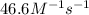
![[a_o]=0.660 M](https://img.qammunity.org/2021/formulas/chemistry/college/tw1nt8s4e97j9bwonyct2pyc0n49n1upll.png)
![[a]=20\% * [a_o]=0.02* 0.660 M=0.0132 M](https://img.qammunity.org/2021/formulas/chemistry/college/v5sarigqux7vp681b3emin1y31yehztorp.png)
t = ?
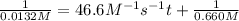
t = 1.59 seconds
It will take 1.59 seconds of time to the concentration of
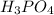 to decrease to 20.0% of its initial value.
to decrease to 20.0% of its initial value.