The mass of 4.5 moles of zinc is 294.21 g.
Step-by-step explanation:
It is known that 1 mole of any element is equal to the molecular mass of that element. For Zinc, it is known that the molecular mass of Zn is 65.38 g. So 1 mole of Zinc will have 65.38 g.
Then 4.5 moles of Zinc will have the mass equal to the number of moles multiplied by the molar mass.
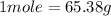
Then,
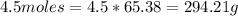
So 294.21 g mass of Zinc is present in 4.5 moles of Zinc.