53 grams of sodium carbonate, 22 grams of carbon dioxide and 9 grams of water.
Step-by-step explanation:
The amount of production of the products from sodium bicarbonate will depend on the molar weight of sodium bicarbonate. The molar weight of a compound is defined as the weight of 1 mole number of molecules of that compound. To determine the molar weight of a compound the easiest process is to add the the atomic weights of individual atoms present in the compound and thus we can obtain the molar weight of the compound.
Molar weight of sodium is 23. The molar weight of hydrogen is 1. The molar weight of carbon is 12. The molar weight of oxygen is 16. So in a molecule of sodium bicarbonate there are one atom of sodium, one atom of carbon, one atom of hydrogen and three atoms of oxygen. So molar weight of sodium bicarbonate becomes
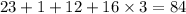 .
.
So 42 grams of sodium bicarbonate contains half mole of sodium bicarbonate.
Molar weight of one mole of sodium carbonate is
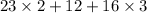 = 106.
= 106.
Molar weight of carbon dioxide is
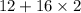 = 44.
= 44.
Molar weight of water is
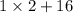 =18.
=18.
Show total amount of products produced will be 53 grams of sodium carbonate, 22 grams of carbon dioxide and 9 grams of water.