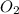Answer: The substance Y in the given equation is
Step-by-step explanation:
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
It means that total number of individual atoms on the reactant side is always equal to the total number of individual atoms on the product side.
For the given chemical equation:
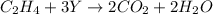
On the reactant side:
Number of carbon atoms = 2
Number of hydrogen atoms = 4
Number of oxygen atoms = 3Y
On the product side:
Number of carbon atoms = 2
Number of hydrogen atoms = 4
Number of oxygen atoms = 6
Evaluating the subscript of oxygen atom:
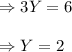
So, the missing substance in the above equation is
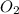
The equation now becomes:
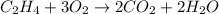
Hence, the substance Y in the given equation is