Answer: The substance is basic in nature because its pH is coming out to be 12
Step-by-step explanation:
pH is defined as the negative logarithm of hydrogen ion concentration present in the solution.
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
We are given:
![[H^+]=10^(-12)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/49mst7a1jfyqy36yubkctc57tzbtb73yfh.png)
Putting values in above equation, we get:
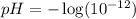
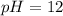
There are three types of solution: acidic, basic and neutral
To determine the type of solution, we look at the pH values.
- The pH range of acidic solution is 0 to 6.9
- The pH range of basic solution is 7.1 to 14
- The pH of neutral solution is 7.
As, the pH of the solution is 12. So, the solution is basic in nature
Hence, the substance is basic in nature because its pH is coming out to be 12