Answer:
(a)
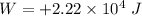
(b)
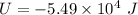
(c)
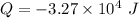
Solution:
As per the question:
Work done,
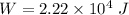
Magnitude of the decrease in the internal energy of the system,
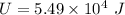
Now,
To determine:
(a) Work done, W:
Since, the work is done by the student on the system, the energy is supplied and as per the sign convention, the work done by the student in this case is positive.
Work done,
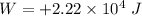
(b) Internal energy, U:
Since, there is a decrease in the internal energy of the system, thus some of the energy has been removed.
Therefore, the decrease in the internal energy is written with negative sign as:
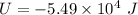
(c) The heat transferred 'Q' is given by the system:
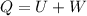
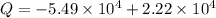
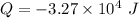
Here, negative sign indicates that heat is evolved and the