Step-by-step explanation:
Let us assume that volumes is equal to spheres volume of nucleus.
fm = femto meter =
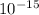
pm = pico meter =
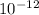
Hence, calculate the volume as follows.
V =
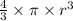
=
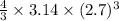
= 82.4481
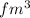 (cubic femtometers)
(cubic femtometers)
or, = 82
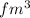
Now, we will calculate the volume of the atom as follows.
V =
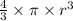
=
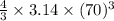
= 1436758.4
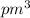 (cubic picometers)
(cubic picometers)
thus, we can conclude that volume of the nucleus is 82
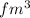 and volume of the atom is 1436758.4
and volume of the atom is 1436758.4
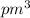 .
.