Answer:
0.025 m
0.059166 m
Step-by-step explanation:
P = Pressure
A = Area
x = Compression of spring
Force is given by
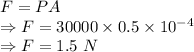
From Hooke's law
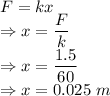
The spring is compressed 0.025 m
In the second case
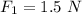
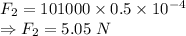
Net force would be
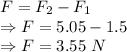
Compression would be
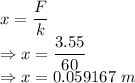
The compression of the spring is 0.059166 m