Answer:
38.32 mL is minimal volume of hot water that is needed to dissolve 2.1 g of pure acetanilide.
Step-by-step explanation:
Mass of acetanilide to be dissolved in hot water = 2.1 g
Mass of hot water to be used = m
Solubility of acetanilide = 55 g/L
This means that 55 grams of acetanilide dissolves in 1 L of hot water.
Then 1 grams of acetanilide will dissolve in :
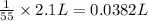
0.0382 L = 0.0382 × 1000 mL =38.2 mL
1 L = 1000 mL
38.32 mL is minimal volume of hot water that is needed to dissolve 2.1 g of pure acetanilide.