Answer:
The 15.230 g of this compound.
Step-by-step explanation:
Given that,
Specific compound = -1250.0 kJ
Molar mass = 82.45 g/mol
Heat =230.90 kJ
We need to calculate the mass
We use the enthalpy change of combustion from kJ/mol to kJ/g.
The compound's molar mass equal to the 82.45 kJ/mol.
One mole of this compound has a mass of 82.45 g
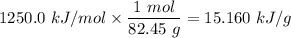
Now, complete combustion in one gram is 15.160 kJ.
As, 15.160 kJ of heat release from 1 gram of compound
So, 230.90 kJ of heat release from
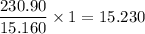 gram of compound
gram of compound
Hence, The 15.230 g of this compound.