Answer:
The final temperature is 25.85°C.
Step-by-step explanation:
Given that,
Mass of copper pot = 0.475 kg
Mass of water = 0.185 kg
Temperature = 19.0°C
Mass of iron block = 0.240 kg
Temperature = 86.5°C
We need to calculate the final temperature
Using formula of heat
Heat lost by iron block=Heat gained by copper pot and water
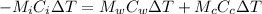
Put the value into the formula
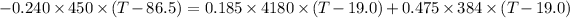
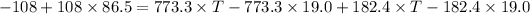
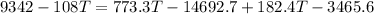
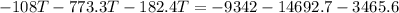
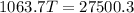
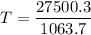
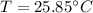
Hence, The final temperature is 25.85°C.