121.2 K
Step-by-step explanation:
P1V1 / T1 = P2V2 / T2
T2 = P2V2T1 / P1V1
P1, V1, T1, P2, V2 are the known quantities
T2 is the unknown quantity.
Standard temperature, T1 = 273 K
Volume, V1 = 2560 ml is converted to litres as 2.56 L
Pressure, P1 = 756 mm Hg is converted into atm as 0.99 atm
P2 = 0.25 atm and V2 = 4.50 L
Now we have to insert these values in the above equation 2 we will get the temperature, T2 as,
T2 =
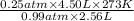
= 121.2 K