Answer:
8 Silicon atom are present in unit cell.
16 oxygen atoms are present unit cell.
Step-by-step explanation:
Number of atoms in unit cell = Z =?
Density of silica = tex]2.32 g/cm^3[/tex]
Edge length of cubic unit cell = a = 0.700 nm =
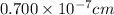
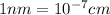
Molar mass of Silica =
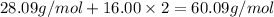
Formula used :
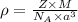
where,
 = density
= density
Z = number of atom in unit cell
M = atomic mass
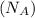 = Avogadro's number
= Avogadro's number
a = edge length of unit cell
On substituting all the given values , we will get the value of 'a'.
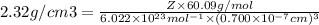
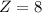
1 silicon is 2 oxygen atoms. then 8 silicon atoms will be 16 oxygen atoms.