Answer:
The answer to your question is 67.8 %
Step-by-step explanation:
Data
62.4 grams of C₂H₅OH
Percent yield
1.- Calculate the theoretical production of Ethanol
1 mol of C₆H₁₂O₆ ------------------ 2 moles of C₂H₅OH
1 mol of C₆H₁₂O₆ ------------------ x
x = (1 x 2) / 1
x = 2 moles of C₂H₅OH
2.- Convert moles to grams
Molecular mass of C₂H₅OH = (12 x 2) + (1 x 6) + (1 x 16)
= 24 + 6 + 16
= 46 g
46 g of C₂H₅OH ---------------- 1 mol
x ---------------- 2 moles
x = (2 x 46) / 1
x = 92 g of C₂H₅OH
3.- Calculate the percent yield
Percent yield =
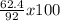
Percent yield = 67.8 %