This is an incomplete question, here is a complete question.
Sodium thiosulfate
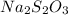 , the major component in photographic fixer solution, reacts with silver bromide to dissolve it according to the following reaction:
, the major component in photographic fixer solution, reacts with silver bromide to dissolve it according to the following reaction:
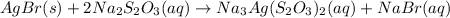
How many grams of sodium thiosulfate would be required to produce 64.3 g NaBr?
Answer : The mass of sodium thiosulfate required would be, 197.6 grams.
Explanation : Given,
Mass of NaBr = 64.3 g
Molar mass of NaBr = 102.9 g/mol
Molar mass of
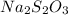 = 158.11 g/mol
= 158.11 g/mol
The balanced chemical reaction is:
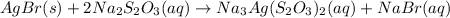
First we have to calculate the moles of
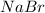 .
.
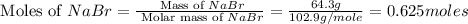
Now we have to calculate the moles of
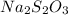
From the reaction, we conclude that
As, 1 mole of
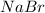 react to give 2 mole of
react to give 2 mole of
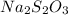
So, 0.625 moles of
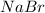 react to give
react to give
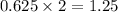 moles of
moles of
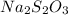
Now we have to calculate the mass of
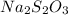
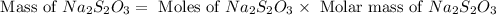
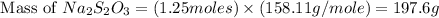
Thus, the mass of sodium thiosulfate required would be, 197.6 grams.